
Scientists Develop Novel "Super-sensitive, Low-burden" Mammalian Cell-cell Communication System
Cell-cell communication, as the "conductor" of physiological activities, transmits information through signaling molecules, coordinating development, immunity, and homeostasis. During embryonic construction, morphogen gradients act as a "GPS," providing cells with precise positional information to guide differentiation and tissue pattern formation. Dysregulation of cell-cell communication systems can lead to severe consequences, including developmental defects or cancer.
Mammalian cell-cell communication systems have been developed based on small molecules such as auxin, but these systems generally suffer from low sensitivity that requires high concentrations of signal and impose a substantial metabolic burden. Besides, they often depend on the external addition of specific chemical precursors to the culture medium, since cells are unable to produce these signals on their own, which limits their applicability in vivo.
In a study published in Cell Systems on Dec. 10, Prof. LOU Chunbo's team from the Shenzhen Institutes of Advanced Technology of the Chinese Academy of Sciences, and collaborators from Peking University, revealed the small-molecule signaling system for mammalian cell-cell communication that combines super-sensitivity with a chassis-friendly, low-burden design, and demonstrated the system's core ability to direct multicellular assemblies in forming complex spatial patterns.
The researchers constructed and optimized a Cinn reception module whose half-maximal effective concentration showed an approximate 375-fold improvement in sensitivity. They defined the minimal core binding sequence of BraR, effectively reducing the risk of potential non-specific binding. They then developed a novel receptor KmaR which maintains super-sensitivity while significantly suppressing basal activity, achieving simultaneous optimization of signal recognition precision and dynamic response range.
For the sender module, the researchers designed a metabolic pathway comprising three enzymes. The pathway utilized phenylalanine to achieve precursor-free, de novo autonomous synthesis of Cinn. The module realized a stable and high-efficiency output of Cinn molecules. Due to the system's high sensitivity, an extremely low proportion of sender cells was sufficient to effectively activate receiver cells, establishing robust cell-cell communication.
To regulate spatiotemporal dynamics of the signal, the system introduced a highly efficient lactonase degradation module to prevent saturation and interference caused by signal accumulation. Evaluations based on cell growth kinetics and transcriptomics indicated no significant differences between engineered cells equipped with these functional modules and wild-type cells, confirming the system's excellent chassis compatibility and low metabolic burden.
The researchers simulated the classic "Source-Sink" spatial gradient pattern model found in biological development. They engineered three types of cells: "Sender" cells (Source) that produce the signal, "Degrader" cells (Sink) that degrade the signal, and "Receiver" cells that sense the signal. The results showed that when the "Source" and "Sink" were positioned at opposite ends of the space, a steep and positionally stable signal response gradient formed in the intermediate region.
This study develops the Cinn system with advantages including precursor independence, super-sensitivity, and low cellular burden, which align perfectly with the requirements of tissue engineering and cell therapy. In the future, researchers plan to utilize this system to design more complex cellular behaviors, or designing intelligent cell therapies capable of sensing the in vivo environment for precise drug release.
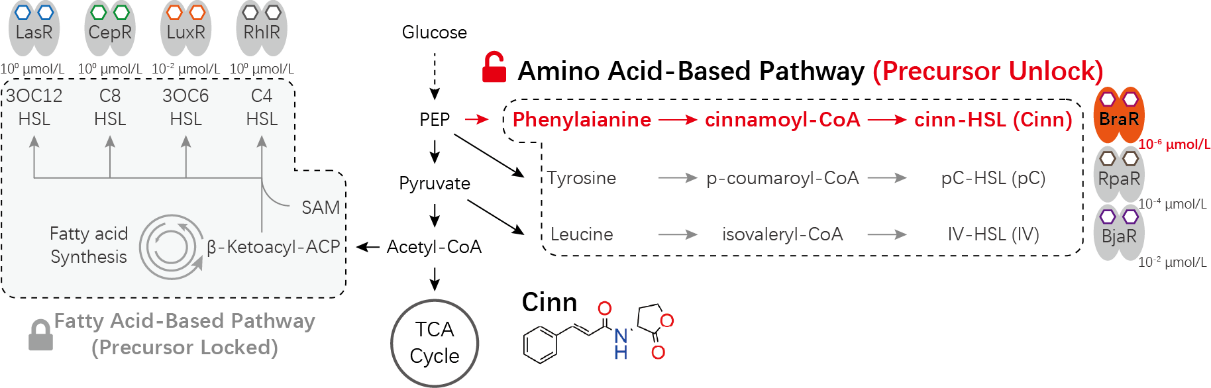
Design Principles of the Novel High-Efficiency Mammalian Cell-Cell Communication Signaling Pathway. (Image by SIAT)
File Download: