Structural Insights into Acarbose Degradation Guide Next-Gen Antidiabetic Drug Design
Type 2 diabetes mellitus (T2DM), a chronic metabolic disorder marked by hyperglycemia, is rapidly becoming a major global health challenge. Acarbose is a potent glycosidase inhibitor widely used in the clinical treatment of T2DM. Howerver, acarbose-preferred glucosidase (Apg) in K. grimontii TD1, significantly degrades acarbose to acarviosine-glucose (M1) and acarviosine (M2), thereby disrupting the efficiency of acarbose.
Recently, a research team led by GU Yang from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Science, ZHOU Jiahai from SIAT and Nanjing Normal University, and ZHANG Jiwen from Northwest A&F University, has conducted an in-depth analysis of the detailed molecular mechanism of Apg hydrolyzing acarbose, providing a crucial structural blueprint for designing a new generation of anti-degradation, more efficient hypoglycemic drugs.
The study was published in Nature Communications on Aug.22.
Previous molecular docking simulations suggested that acarbose was hydrolyzed by orienting the nucleophile D336 towards at the linkage between the third and the fourth rings, yielding the three-ring product M1, or at the linkage between the second and the third rings, yielding the two-ring product M2 (Nat. Metab. 2023, 5, 896).
In this study, the researchers combined high-resolution structural analysis, biochemical experiments, and multiscale computational simulations to comprehensively elucidates the molecular mechanism by which Apg degrades acarbose. They first identified D448 as the key nucleophile, rather than D336 as previously assumed. Then they revealed that both E373 and R334 can function as substrate donors. Their findings demonstrate that acarbose degradation proceeds via a two-step mechanism involving an M1 intermediate, with the second-step hydrolysis being the rate-limiting step.
Moreover, leveraging the crystallographic structures, the researchers conducted comprehensive computational evaluations of the acarbose analogs acarstatins A and B, demonstrating their resistance to Apg. These findings provide clear targets for designing novel anti-degradation diabetes therapeutics—specifically, by interfering with the nucleophilic attack of D448 through extension of the sugar chain or modification of the -1 subsite.
This study provides structural insights that inform the design and development of next-gen antidiabetic drug.
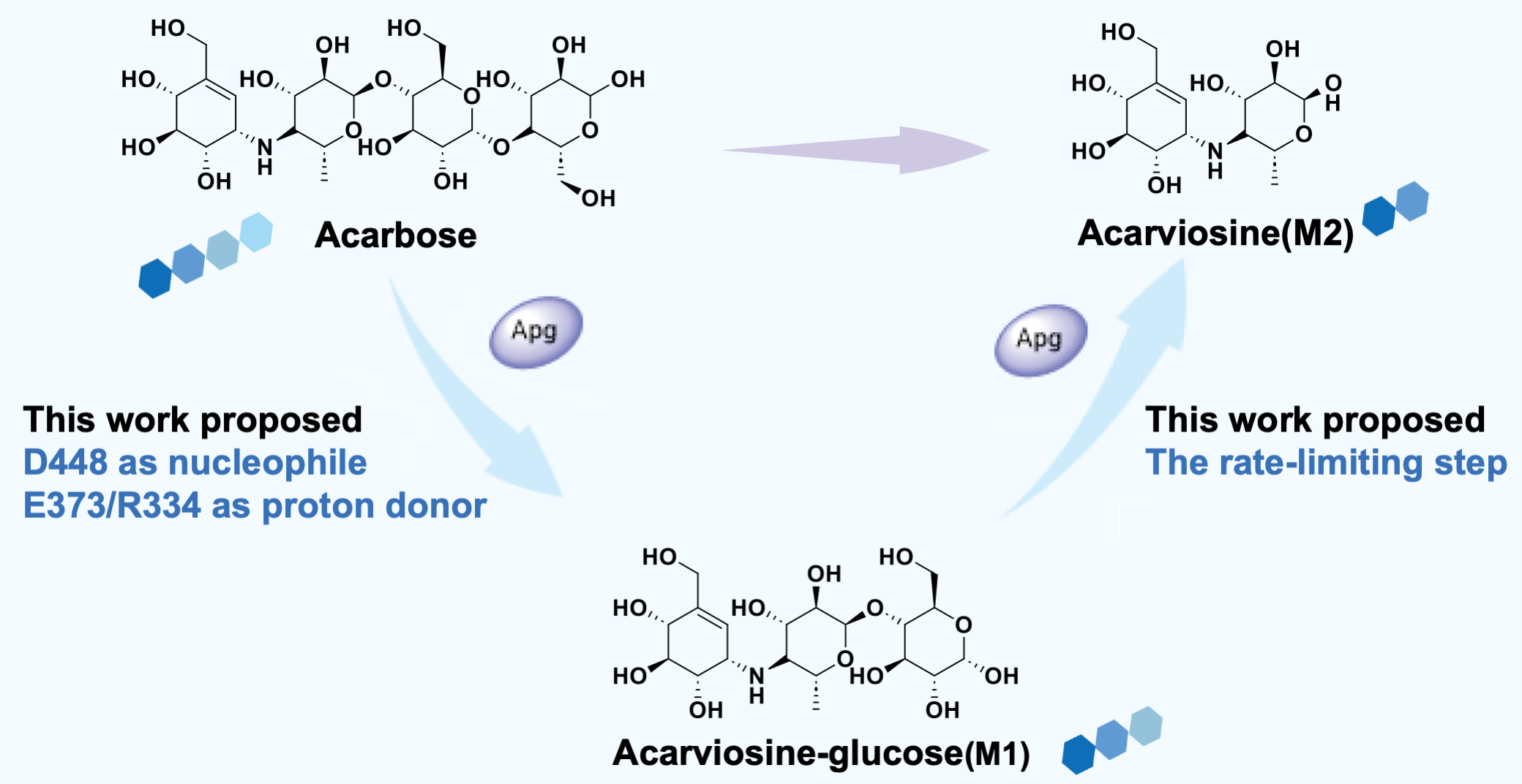
Mechanism of Apg-catalyzed hydrolysis of acarbose. (Image by SIAT)
File Download: