Targeting "Aged" Immune Cells: A New Strategy to Boost Immunotherapy in Solid Tumors
Head and neck squamous cell carcinoma (HNSCC) is a malignant tumor originating from the epithelial cells of the head and neck region. As a neoadjuvant treatment for locally advanced HNSCC, chemoimmunotherapy has significantly improved pathological response rates. Yet, its efficacy is constrained by three major challenges: variable patient responses, overlapping treatment-related toxicities, and the emergence of drug resistance. Balancing long-term benefit with quality of life remains a major clinical hurdle.
In a study published in Nature Medicine on August 25 has revealed immunosenescence in the tumor microenvironment as a key mechanism contributing to this challenge, and showed that targeting senescent immune cells can improve the effectiveness of immunotherapy.
This study was conducted by a collaborative team led by Prof. FAN Song from Sun Yat-sen Memorial Hospital, Prof. FAN Xiaoying from Sun Yat-sen University (SYSU), Prof. FAN Xiaoying from Guangzhou National Laboratory, Assoc. Prof. XU Fang from the Shenzhen Institutes of Advanced Technology (SIAT) of Chinese Academy of Sciences, and Prof. WANG Xinhui from Harvard Medical School (HMS).
The researchers began with the oral/oropharyngeal squamous cell carcinoma (OOC-001) phase II clinical trial, in which 51 patients with resectable HNSCC received neoadjuvant chemoimmunotherapy. While some achieved complete pathological response, a subset showed no benefit. Using single-cell multi-omics profiling, the researchers found that non-responding tumors exhibited prominent features of immunosenescence, including reduced CCR7+ CD4+ naïve T cells and CD27+ memory B cells.
Building on these insights, they tested senolytic drugs in animal models. The combination of dasatinib and quercetin (D+Q) with PD-1 blockade significantly reduced tumor burden and extended survival, surpassing the efficacy of PD-1 inhibitors alone or in combination with chemotherapy. Importantly, the senolytic combination restored naïve T cell function and reversed markers of immune aging.
Encouraged by these results, the researchers launched the world's first phase II clinical trial (COIS-01) testing senolytics plus immunotherapy. Twenty-four patients with resectable HNSCC received neoadjuvant treatment with tislelizumab (anti–PD-1), dasatinib, and quercetin. The regimen achieved a 33.3% major pathological response rate with markedly lower toxicity compared with chemoimmunotherapy. Notably, only one patient experienced grade 3–4 adverse effects, versus more than half in the chemoimmunotherapy cohort.
"Our study provide a new strategy for solid tumor immunotherapy," the authors noted. "Moving forward, we will focus on developing more potent senolytics, refining treatment regimens, and expanding clinical applications."
By identifying immunosenescence as a key barrier to immunotherapy and providing a viable strategy to overcome it, this study represents a conceptual and therapeutic breakthrough with broad implications for oncology.
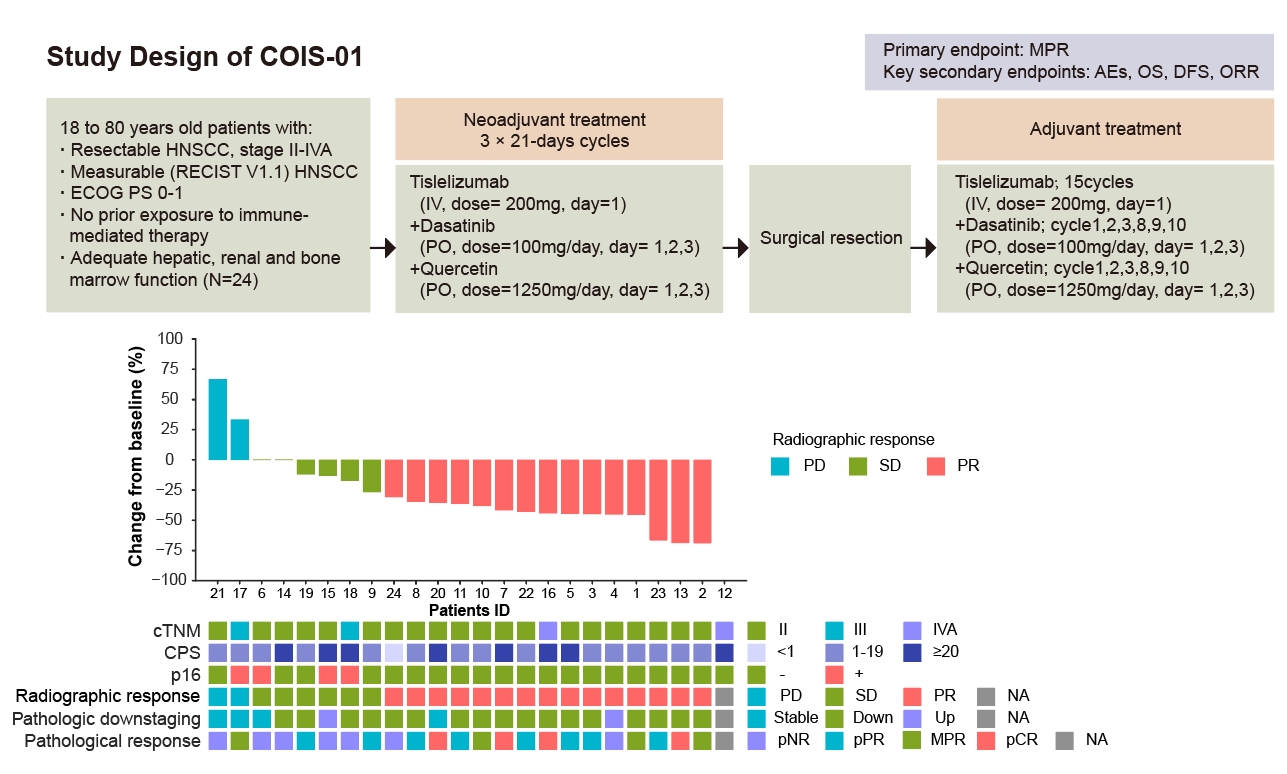
Study design and patient characteristics from the COIS-01 Trial. (Image by SIAT)
File Download: