Scientist Develop New Method Prepare Na0.44MnO2/rGO for Sodium-Ion Batteries
Date:06-06-2019 | 【Print】 【close】
Secondary batteries have a wide range of applications in rechargeable electronic devices, and lithium-ion batteries (LIBs) are currently the mainstream technology due to their high energy density, lightweight, small volume and long service life.
Nevertheless, with the rapid development of large-scale renewable energy storage and electrical grid applications, further research and development of LIBs are confronted with the short supply of lithium resources.
There is tremendous interests in alternative electrochemical energy storage systems. Owing to the similar electrochemical properties between Li+ and Na+, abundant supplies of sodium resources as well, low-cost sodium-ion batteries (SIBs) are explored as one of the most promising candidates to partly replace LIBs, and it has potential for large-scale renewable energy storage applications.
A research team led by Dr. FU Bi at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, in collaboration with SU Yong and YU Junxi from Xiangtan University, developed the single crystalline nanorods of Na0.44MnO2 enhanced by reduced graphene oxides (rGO) as a high rate and high capacity cathode materials for SIBs.
Na0.44MnO2/rGO nanocomposite was synthesized by a simple wet-chemistry rote. When used as a cathode material for SIBs, Na0.44MnO2/rGO nanocomposite exhibits reversible specific capacity of 124mAh/g at 0.2C after 200 cycles and 70.8mAh/g at 15C, which are significantly enhanced over control cathode made of pure Na0.44MnO2 nanorods. More importantly, the electronic conductivity of Na0.44MnO2/rGO nanocomposite was enhanced by one order of magnitude using rGO, as quantified by local conductive atomic force microscopy (c-AFM) measurement at the nanoscale. Through the detailed electrochemical impedance spectroscopy (EIS) analysis, it was found that Na0.44MnO2/rGO nanocomposite exhibits much reduced Warburg factor, resulting in enhanced Na+ diffusion at each discharging voltage platform.
This suggests that the enhanced electronic conductivity helps Na+ insertion/deinsertion, resulting in improved electrochemical performance, and these insights help us understand how electronic conductivity improves ionic transport kinetics in SIBs.
The relevant research entitled “Single crystalline nanorods of Na0.44MnO2 enhanced by reduced graphene oxides as a high rate and high capacity cathode material for sodium-ion batteries” was published in Electrochimica Acta.
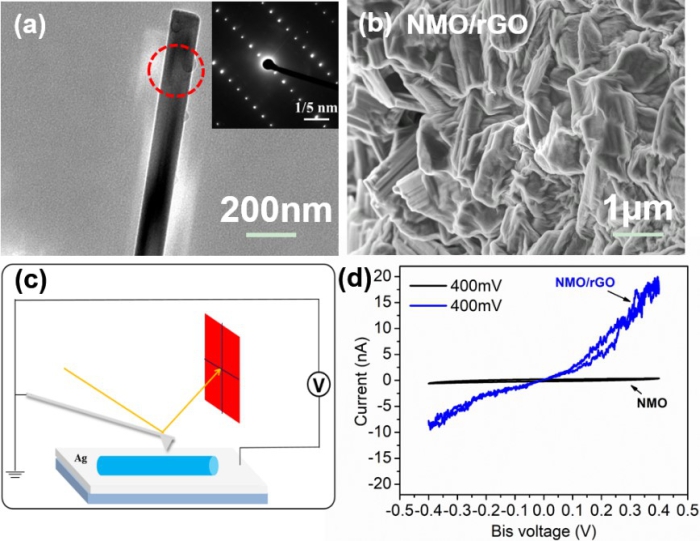
Fig. (a) TEM image of a single NMO NR and the corresponding SAED pattern in the inset; (b) SEM image of NMO/rGO; (c) The schematic diagram of c-AFM measurement; (d) the local I-V curves of NMO/rGO and NMO under a bias voltage of 400 mV.
CONTACT:
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn
Tel: 86-755-86585299
