Researchers Use Chemical Modification Improves Biocompatibility of Black Phosphorus Nanosheets
Date:22-09-2017 | 【Print】 【close】
Black phosphorus (BP), the most stable allotrope of the phosphorus element, is presently attracting intense research interests as a novel 2D material in the worldwide. In recent years, few-atomic-layer of BP (BP nanosheets, BPs) have been produced by exfoliation techniques, and exhibit many unique properties such as high mobility, highly anisotropic charge transport, and distinct optical response properties. As a result of these unique characteristics, BPs have been regarded as a promising nanomaterial not only in nanoscale electronic and optoelectronic devices, but also in various biomedical applications. Typically, BPs with excellent near-infrared photothermal and photodynamic performances have exhibited great potential in cancer therapy. Combined with their phototherapy abilities, BPs have also been successfully applied as sensitive in vivo photoacoustic imaging agents. Furthermore, ascribed to their atomically thin 2D structure and large surface area, BPs have been proposed as efficient drug delivery platforms, and multifunctional theranostic agents in the treatment of cancer. However, the actual biological effects of few-layered BPs as nanomaterials are difficult to predict in vivo, and the understanding towards the biological effects of BPs is extremely limited.
As an important part of immune system, mononuclear phagocyte system (MPS) plays a crucial role in the clearance of nanomaterials from the circulation upon medical application. In recent years, various nanomaterials have been investigated and reported as active foreign particles that can stimulate inflammatory effects when administered to animals or humans. Severe or sustained inflammatory responses can lead to diseases due to damages to normal organs or tissues by the stimulated immune cells. Therefore, the reduction of macrophages uptake of the injected nanomaterials has been regarded as an important strategy to enhance corresponding therapeutic efficacy and reduce the potential toxicity caused by their nonspecific deposition in MPS. As a new biomaterial with great application potential, the inflammatory effects of BPs no doubt deserve detailed investigation. The corresponding strategy to improve their biocompatibility is also highly desired, not only in terms of environmental exposure but also as a mean to reduce the toxicity for biomedical uses.
The research group led by Prof. YU Xuefeng, from Shenzhen Institutes of Advanced Technology, Chinese Academic of Science, and the research group led by Prof. JIANG Guibin, from Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, conducted in vitro and in vivo experiments were performed to investigate the inflammatory effects and biocompatibility of ultra-small BPs (BPQDs) (named Bare BPs) and ultra-small BPs with titanium sulfonate ligand (TiL4) modification (named TiL4@BPs)). BPs was synthesized using a liquid exfoliation technique in N-methyl-2-pyrrolidone (NMP), then mixed with TiL4 in NMP at room temperature for 15 h to produce TiL4@BPs. The morphology of TiL4@BPs was examined using transmission electron microscopy (TEM), high-resolution TEM (HR-TEM), and atomic force microscopy (AFM). As shown in Figure 1, TiL4@BPs had an average size and height of about 3.3 nm and 1.5 nm, respectively. The HR-TEM image shows their lattice fringes of 0.21 nm, corresponding to the (014) plane of the crystal of BPs.
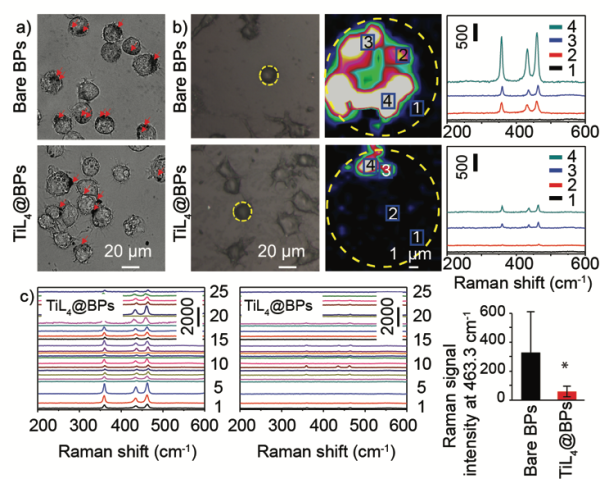
It should be noted that such chemical modification did not change the crystal morphology and structure of the BPs. High-resolution X-ray photoelectron spectroscopy (HR-XPS) was performed to assess the chemical quality of bare BPs and TiL4@BPs. As shown in Figure 2, bare BPs exhibited the P2p3/2 and P2p1/2 doublet at the 130.1 and 130.9 eV respectively, characteristic of crystalline BPs, and intense oxidized phosphorus sub-bands were apparent at 134.0 eV as a result of partial oxidation. In contrast, the binding energy of P2p in TiL4@BPs was 132.4 eV, in accordance with the reported value of Ti-P coordination, and oxidized phosphorus sub-band was not found. The XPS thus confirmed successful coordination between P and Ti in TiL4@BPs. Furthermore, it was found that the zeta potential changed from −36.5±1.1 mV in bare BPs to +21.1±2.6 mV after TiL4 coordination in TiL4@BPs .
In addition, TiL4@BPs exhibited higher stability against oxidation and degradation than bare BPs and the corresponding mechanism has been reported previously.
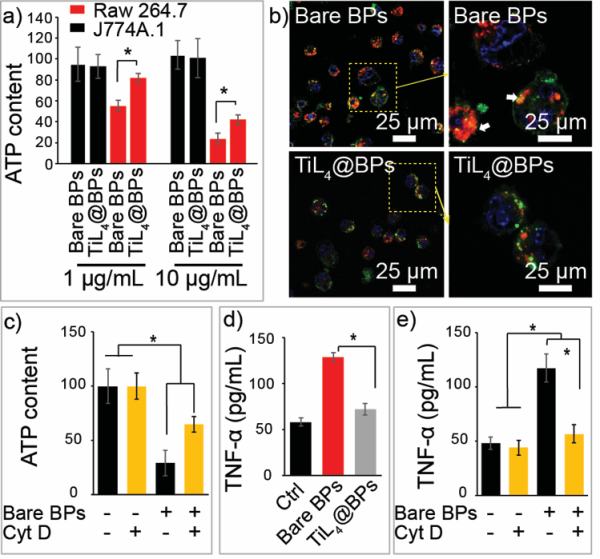
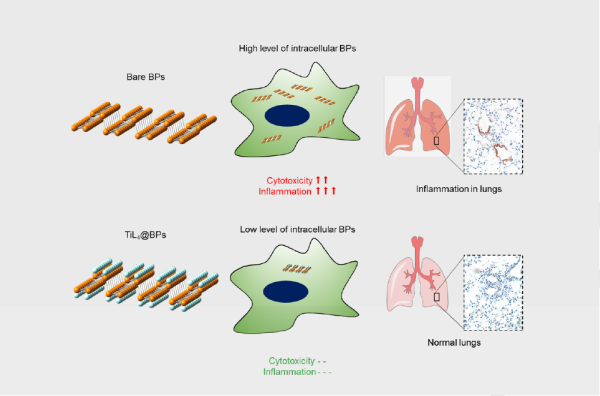
In conclusion, in vitro and in vivo biological effects of BPs with and without TiL4 modification have been investigated in detail. In vitro experiments demonstrated that BPs with TiL4 modification can escape the cell uptake by macrophages, and further reduce the cytotoxicity and proinflammation. When intravenously injected into mice, bare BPs trigger significant inflammatory responses, including an increase in peripheral neutrophils accompanied by elevation of a group of inflammatory cytokines. In contrast, TiL4@BPs exhibit no such adverse immune responses. The mechanisms of the BPs’ inflammatory effects and the improved biocompatibility caused by the TiL4 modification have been discussed. The detailed investigation of biological effects of BPs may guide their applications as foreign particles administered to animals or humans. In addition, our findings proposed an efficient strategy to improve the biocompatibility of BPs, and the synthesized TiL4@BPs may have a great potential in biomedical applications.
The paper titled “Chemical modification improves biocompatibility of black phosphorus nanosheets” was published on Angewandte Chemie International Edition. Prof. YU Xuefeng and Prof. JIANG Guibin are the corresponding authors. This work was supported by grants from the National Natural Science Foundation of China , the Youth Foundation of Chinese Academy of Inspection and Quarantine , Frontier Research Key Project of the Chinese Academy of Sciences, and Leading Talents of Guangdong Province Program.
Contact:
Prof. YU Xuefeng
Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences
Tel: 00-86-755-86392212
Email: xf.yu@siat.ac.cn
