Can the Host Run Fast Enough to "Eliminate" the Virus?
Date:04-12-2024 | 【Print】 【close】
A research group led by Prof. FU Xiongfei at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences has developed a synthetic host-virus co-propagation system that demonstrates chemotaxis-mediated range expansion can contribute to the suppression of infectious disease, independently of intricate host-virus dynamics and specific environmental conditions.These results challenge the notion that host migration speeds up virus transmission, instead showing how it can suppress virus spread.
The study was published in the Proceedings of the National Academy of Sciences (PNAS) on Dec. 3.
Species expanding into new areas often carry multiple zoonotic pathogens, emphasizing the need for human attention. Therefore, studying virus transmission in expanding host populations is crucial for predicting future infection risks to wildlife, plants, and humans. The role of host mobility in virus transmission has been debated, with a common perspective being that host movement tends to accelerate the spread of virus. However, numerous studies have indicated that host migration might actually suppress virus spread.
So how exactly does host movement influence virus transmission? Previous studies on the spatio-temporal dynamics of virus transmission have primarily relied on epidemiological data, with resultant theories lacking sufficient experimental validation.
In this study, researchers constructed a bacteria-bacteriophage co-propagation system using E. coli and its virus, M13 phage, to investigate how host range expansion affects the relationship between host movement and virus distribution. They found that under unguided range expansion, following the canonical Fisher–Kolmogorov dynamics, viral spread increased with the speed of bacterial migration. In contrast, during chemotaxis-driven navigated range expansion, viral spread decreased.
Theoretical and experimental studies showed that bacteria's chemotactic migration significantly inhibits virus spread. Faster migration reduces viral spread, and at high speeds, infected individuals are completely cleared from the group, a process known as "migratory culling".
Theoretical predictions revealed that the spatial segregation of uninfected and infected hosts within the propagating front speeds up the back diffusion of infected individuals. Increasing the migration speed can remove infected cells from the front. Furthermore, fluorescent labeling experiments also showed the distinct distribution of uninfected and infected cells at this front.
"Our work links established molecular and cellular knowledge with predictive host-viral dynamics during migration, providing a robust framework for ecological systems biology through integrated experimental and theoretical approaches," said Prof. FU.
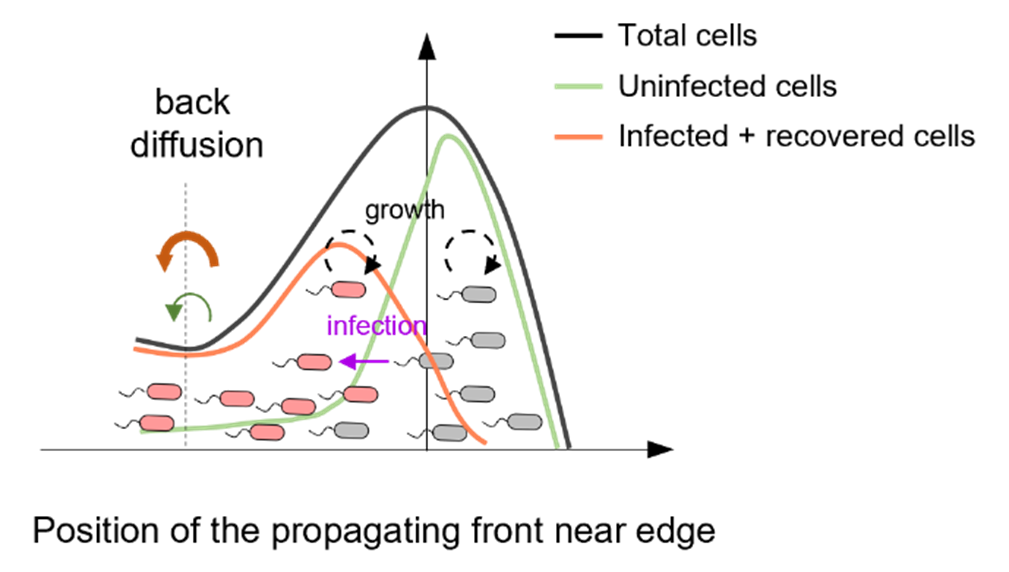
Illustration of the spatial sorting mechanism for migratory culling. (Image by SIAT)
Media Contact: LU Qun
Email: qun.lu@siat.ac.cn
Download the attachment: