Researchers Develop Dissipative Droplet Systems Capable of Chemotactic Movement
Date:11-11-2024 | 【Print】 【close】
Life maintains its structures and functions through energy consumption, operating far from equilibrium. Dissipative chemical processes, due to their potential for far-from-equilibrium properties and functions, have attracted significant attention in the development of life-like materials and systems. These processes transform energy from one form to another. In recent years, chemists have sought to harness these processes to design systems with specific functions.
However, achieving mechanical functions through dissipative self-assembly, similar to those found in biological structures like microtubules, remains a significant challenge.
Recently, Prof. LIU Kai from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, in collaboration with a research team led by Prof. Sijbren Otto from the University of Groningen, has developed an active droplet system that can exhibit chemotactic movement through the cross-scale conversion of energy.
This study was published in Nature Chemistry on Nov. 8.
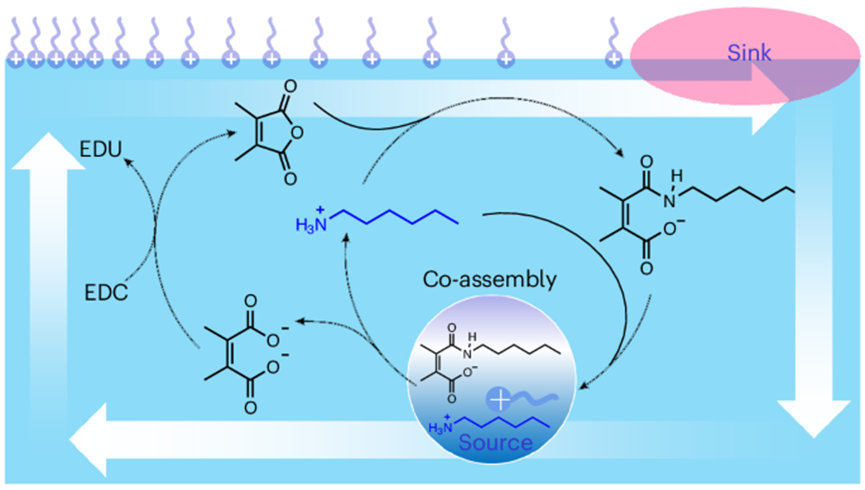
In this study, researchers created the active droplets by employing a dissipative reaction network that involves a dynamic amide bond, which is formed by mixing maleic anhydride and octylamine in an aqueous solution. This bond is also readily hydrolyzed under acidic conditions. Carbodiimide serves as a secondary fuel molecule, driving the reformation of the amide compound from the diacid waste and octylamine, thus establishing a dissipative cycle.
In this process, the amide product and octylamine co-assemble into droplets through intermolecular electrostatic and hydrophobic interactions. The dynamics of this self-assembly can be tuned by the addition of chemical fuels, resulting in transient growth of the droplets.
When oleic acid is added to the surface of the droplet solution, the droplets on the air-water surface move towards the oleic acid. This behavior is resulting from the coupling between the dissipative self-assembly process and the Marangoni effect, which induces Marangoni flow that propels the movement of the droplets. In addition, the speed and duration of the droplets' motion can be precisely regulated.
In this system, chemical fuels drive the formation of amide bonds at the molecular level, while at the nanoscale, they promote the creation of high-energy active droplets. On the macroscopic scale, these fuels enable the directed movement of the droplets.
"Our study demonstrates that high-energy dissipative assemblies can function as energy converters, which may open up new perspectives for the development of active materials, such as micro-nano motor, soft robot and non-equilibrium pattern," said Prof. LIU. "Moreover, the proposed droplet system, composed of simple molecules, could serve as a model for primitive cells, capable of exhibiting complex collective behaviors."
Active droplets drive Marangoni flow. (Image by SIAT)
Media Contact: LU Qun
Email: qun.lu@siat.ac.cn
Download the attachment: