Complex Biological Behaviors: How Multiple Oscillators Interact In Live Cells
Date:19-05-2023 | 【Print】 【close】
In 1665, Christian Huygens observed the synchronization of two pendulum clocks and documented his findings. Oscillatory dynamics, observed not only in physical systems but also in fundamental biological processes like circadian clocks, segmentation, and transcription factor responses, requires precise quantitative control for proper cell regulation and fate decisions. Many biological oscillators are influenced by multiple oscillatory signals, and their behavior is understood through the theory of Arnold Tongues. However, this theory simplifies the situation to a single external signal and one internal oscillator, which oversimplifies real biological systems. Our understanding of how an oscillator responds to two or more external oscillatory signals is currently insufficient.
To address this longstanding puzzle, a joint research team from Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences and Niels Bohr Institute of Copenhagen University constructed a synthetic oscillatory system in yeast that can response to dual oscillatory signals. By tightly integrating experiment and mathematical modeling, they have revealed the cooperative effect of multiple oscillatory signals and the unique phase regulation phenomenon. These findings suggested a novel path to control oscillatory dynamics in both natural and synthetic biological systems.
This work was published in Cell Systems on May 17.
The researchers modified a previously constructed synthetic oscillator in yeast to acquire a dual responsive oscillator. They validated the system can be synchronized by both periodic α-factor and ethanol, thus representing a tri-coupled oscillatory system. A mathematical model was also derived to fit the parameters and predicting further outcomes.
Combining modeling and experimental approach, the researchers found that two oscillatory signals together can significantly enlarge the entrainment region, increase the ratio of synchronized cells, and delay the onset of chaos. These results suggest that two oscillatory signals were not linearly combined, but in cooperation to stabilize the synchronization.
"I had not heard of such a surprising phenomenon before and it is quite interesting that is unveiled in a biological context. Moreover, evidence for this phenomenon is provided both theoretically and experimentally, using a synthetic circuit, which is an impressive tour de force." Said one of the anonymous reviewers of the paper.
One step further, the researchers discovered that the phase difference between two external oscillatory signals was a critical parameter for the dynamics of the internal oscillator. By tuning this phase difference, one may finetune the internal oscillation amplitude but remain the oscillation frequency constant. The researchers further revealed that the optimal phase difference is tightly related to the natural phase difference of the different components of the system in free-oscillating condition. To validate the universality of the results, the authors also modeled three other oscillatory systems, including the van de pol oscillator, the p53 oscillator and the natural NF-κB oscillator. In all three systems tested, the core findings were shown to be robust and consistent.
As a final validation of the phase regulation mechanism, the researchers asked whether tuning phase difference of oscillatory signals could affect downstream gene transcription. Despite the existence of noise, they found that gene expression level is indeed significantly affected by phase modulation, and this is further linked to the different amplitudes of the internal oscillator.
"Our novel synthetic cell signaling system, together with mathematical modeling, serve as a powerful platform to study such complex biological problems." Said Prof. WEI Ping, one of the corresponding authors, "The results presented here broadened our general understanding of biological coupled oscillators and emphasized the importance of a correct time pattern in biological regulation. We hope that these findings may inspire scientist from the much more broad disciplines."
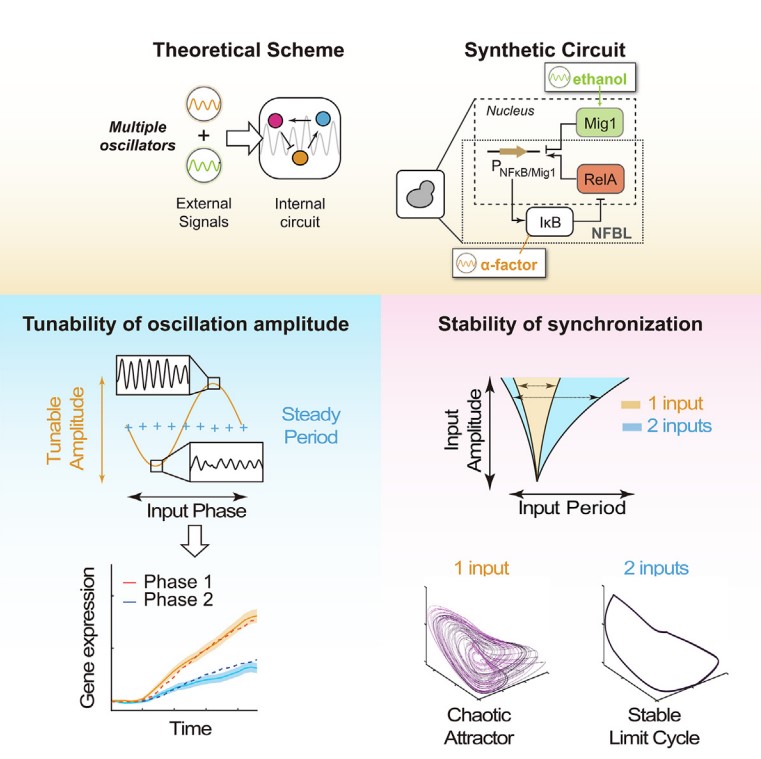
Working model outlying how three oscillations interplay and perform new dynamic behaviors. Left panel: The amplitude-only modulation of the internal oscillator enabled by tuning the relative phase of two oscillatory signals. Right panel: The stability of the entrainment is enhanced by additional external oscillations. (Image by Prof. WEI Ping)
Media Contact:
ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn