Researchers Revealed Molecular Mechanism of Pannexin 2 as an ATP Membrane Pore Channel
Date:09-03-2023 | 【Print】 【close】
Recently, a research team collaborated by Prof. YUAN Shuguang from Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences and Prof. ZHANG Huawei from the South University of Science and Technology revealed the molecular mechanism of pannexin 2 (Panx2) as an ATP membrane pore channel.
This work, which was entitled "Cryo-EM structure of human heuristic pannexin 2 channel", was published in Nature communications on March 3rd.
ATP membrane pore channel protein plays an important physiological role in human physiological processes, including immune regulation, energy generation, and cancer. The abnormal function of ATP membrane pore channel membrane protein leads to severe consequences such as ischemic cerebral infarction, glioma, and pleomorphic malignant glioma. Taking brain glioma as an example, the total survival time of patients with a higher level of Panx2 is longer, which suggests that Panx2 may have an anti-tumor effect in the early stage of glioma.
Pannexins protein family, which includes Panx1, Panx2, and Panx3, can form macroporous non-selective transmembrane (TM) channels. They play an important role in cell communication and homeostasis. Panx2 protein is the largest Pannexins family member. It is mainly expressed in the central nervous system. The team solved the high-resolution structure of the Panx2 membrane protein through the freezing electron microscope. It found that Panx2 is a four-TM domain protein with seven monomer proteins gathered together, forming a transmembrane pore. By comparing the structures of Panx2 and Panx1, the team speculated that Panx2 might be the channel of ATP.
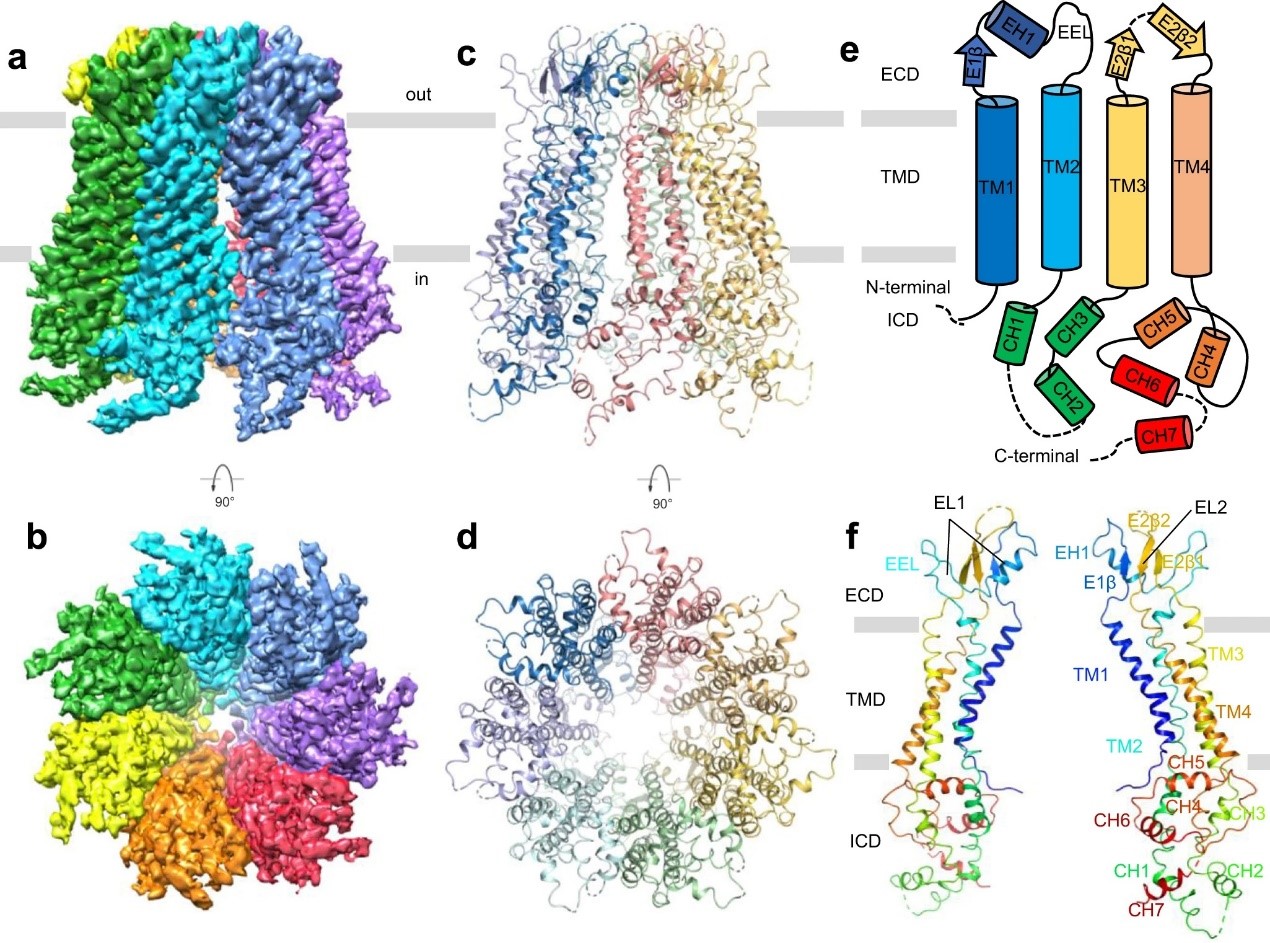
Figure1. Cryo-EM structure of the human Panx2 channel. (Image by SIAT)
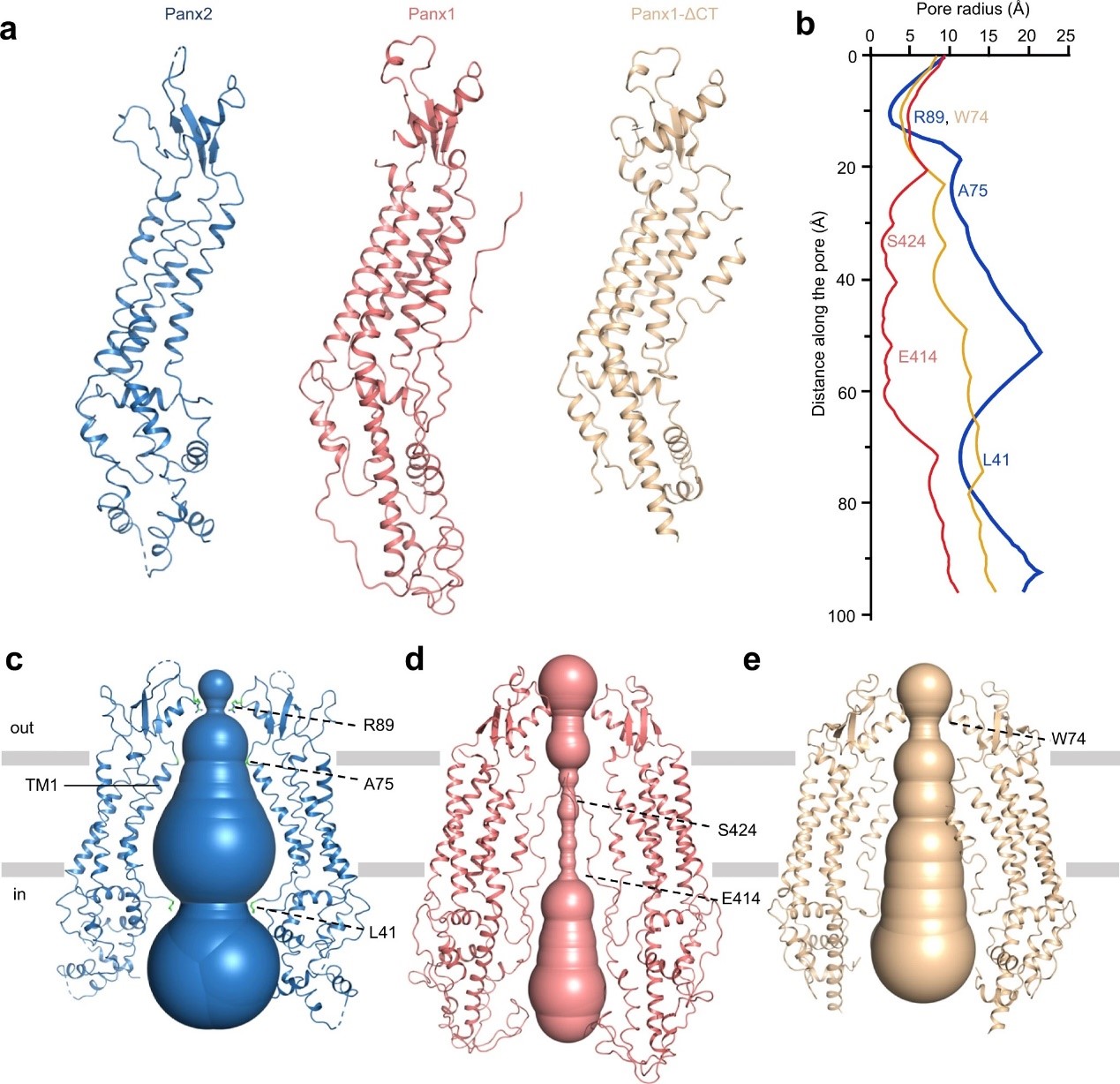
Figure 2. Comparison of the structures of Panx2 and Panx1. (Image by SIAT)
The team then verified the above hypothesis through ATP release assay and molecular dynamics simulation. In the ATP release assay, the team found that the efficiency of Panx2-NT-R89A was significantly higher than the counterpart of the wild-type Panx2. This resulted implied that amino acid R89 is responsible for ATP passing through Panx2. In addition, molecular dynamics simulation showed that the side chain of R89 swings flexibly, resulting in the pore size of the channel increases accordingly. Such changes corresponds to the diffusion of ATP.
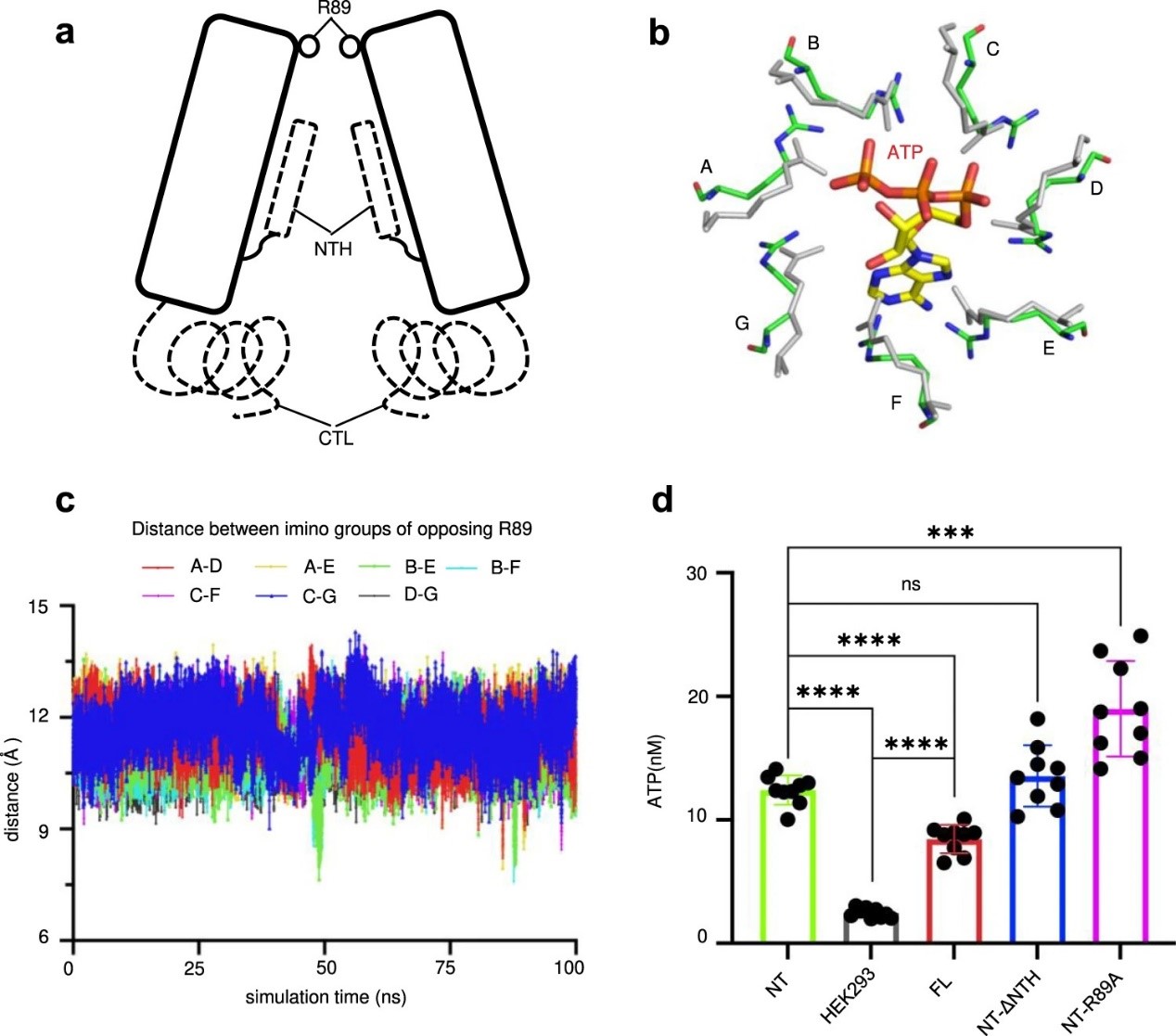
Figure 3. The Panx2 channel is gated by R89. (Image by SIAT)
"Our work illuminated the 3D structures of Panx2 transmembrane protein at the atomic level," said Prof. YUAN, "it helps to understand the fundamentally biological function of Panx2 which provides an insightful view into related drug discovery as well."
Media Contact:
ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn