A Low-Cost and Environmental Friendly Mixed Polyanionic Cathode for Sodium-Ion Storage
Date:12-11-2019 | 【Print】 【close】
Recently, Prof. TANG Yongbing and his team members from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, along with the National Institute of Synchrotron Radiation Sources of Thailand, have developed a low-cost and environmental friendly mixed polyanionic cathode for sodium-ion storage.
This work is of great significance for the development of advanced electrode materials and energy storage devices. The relative research “A low-cost and environmental friendly mixed polyanionic cathode for sodium-ion storage” has been published online in Angew. Chem.
Traditional lithium ion batteries (LIBs) have been widely used in portable electronic equipment, electric vehicles and other fields. However, LIBs are hard to meet the demand of large-scale energy storage applications because of the uneven distribution and limited lithium resources, as well as the scarcity of cobalt resources used in LIBs cathode.
Due to the similarity between sodium and lithium, the rich abundance of sodium, sodium ion batteries (NIBs) become promising alternative of LIBs in the field of large-scale energy storage. However, the application of NIBs is limited by lacking of suitable and cheap cathode materials. The main reason is that since sodium ion has a larger ion radius than lithium ion, it is relatively difficult for sodium ion to desert/insert from/into the positive electrode material, which requires a more stable crystal skeleton and larger ion diffusion channels.
Considering above situations, Prof. TANG Yongbing and his team members (SONG Tianyi, Dr YAO Wenjiao, Dr ZHENG Yongping etc.) successfully developed a new mixed polyanionic compound, Na2Fe(C2O4)SO4·H2O, as a cathode for NIBs, via experimental and simulation methods. This cathode exhibits excellent electrochemical reversibility and stability. Its electrochemical mechanism was further demonstrated to be originated from the redox of Fe2+/Fe3+ through in-situ synchrotron X-ray measurements and DFT calculations. Simulation also revealed that the high stability is owing to the large size of sodium ion migration channels and its rigid three-dimensional framework. This work has provided an excellent case in search of novel NIBs cathode among mixed polyanionic families and may promote the development of high efficient and low-cost energy storage technology.
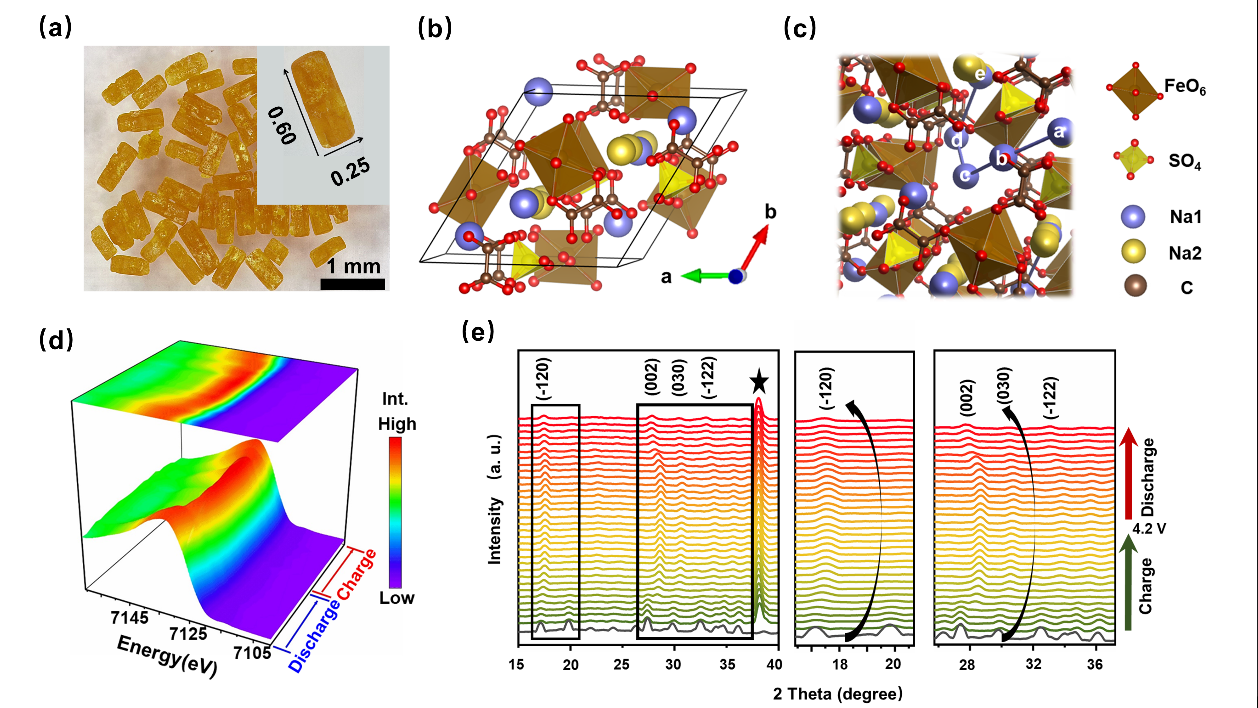
Figure. Characterization of sodium ion cathode material. (a) Single crystallites from hydrothermal method (b) Structure in a unit cell. (c) Schematic illustration of ion diffusion pathway from a-e. (d) Synchrotron X-ray absorption spectra of Fe K-edge (e) In-situ XRD spectra in a cycle.
CONTACT:
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn
Tel: 86-755-86585299