Co Doped Phosphorene: An Effective Hydrogen Evolution Electrocatalyst
Date:06-05-2019 | 【Print】 【close】
Researchers have developed a new approach to synthesize Co doped few-layer phosphorene, an effective water splitting electrocatalyst to generate hydrogen which is considered as an ideal alternative fossil fuels to retard environmental pollution.
A research team led by Prof. YU Xuefeng, in collaboration with LIU Danni and Dr. WANG Jiahong at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, developed a facile and rapid electrochemical method to synthesize Co doped few-layer phosphorene with enhanced hydrogen evolution reaction activities, this sort of phosphorene is more stability than the bare phosphorene.
The relevant researches, named Direct Synthesis of Metal‐Doped Phosphorene with Enhanced Electrocatalytic Hydrogen Evolution, were published in Small Methods.
Two-dimensional (2D) materials possess great potential in electrocatalysis, with the advantages of unique atomic thickness, ultrahigh surface area, and specific charge-carrier confinement. As an emerging class of 2D materials, few-layered black phosphorus (BP), also named phosphorene, has been proposed to be a promising electrocatalyst on account of the large exposed surface and high carrier mobility.
This method enables synchronous exfoliation and metal doping to directly convert bulk black phosphorus to transition metal-doped phosphorene based on the synergistic effect among intercalator molecular (TBA+), metal ions (Co2+), and the solvent (DMF). TBA+ and Co2+ in the electrolyte migrate to the bulk BP and the flexible hydrocarbon chains of TBA+ enable intercalation of bulk BP and expansion of the interlayer.
At the same time, n-Bu3N decomposed from TBA+ reacts with Co2+ and DMF to form the cationic Co-Bu3N-DMF complex which got into the interlayer along with free TBA+. Co2+ from Co-Bu3N-DMF complex is trapped by the negatively charged phosphorene and reduced into an atomic dopant on the surface. Additionally, the coulomb interaction and steric effect among the Co-Bu3N-DMF complex made them repulsed each other, and prevented the aggregation of the Co atoms. When the interlayer accumulates enough molecules, the bulk BP crystal expands into a sponge which is a cross-linked structure of the BP (Co) products. This method can be extended to synthesize various transition metal doped few-layer 2D sheets.
Owing to the enhanced electro-conductivity, abundant metal-P active sites, and optimized adsorption energy by doping, the BP (metal) exhibits enhanced hydrogen evolution reaction activities and stability in comparison to the bare phosphorene. Particularly, BP (Co) presents highest activity with a potential of 0.294 V at 10 mA cm-2.
This effective and massive production strategy offers an extendable availability for 2D electrocatalysts, as well as provides a solution for structure modulation and adatom doping of 2D materials.
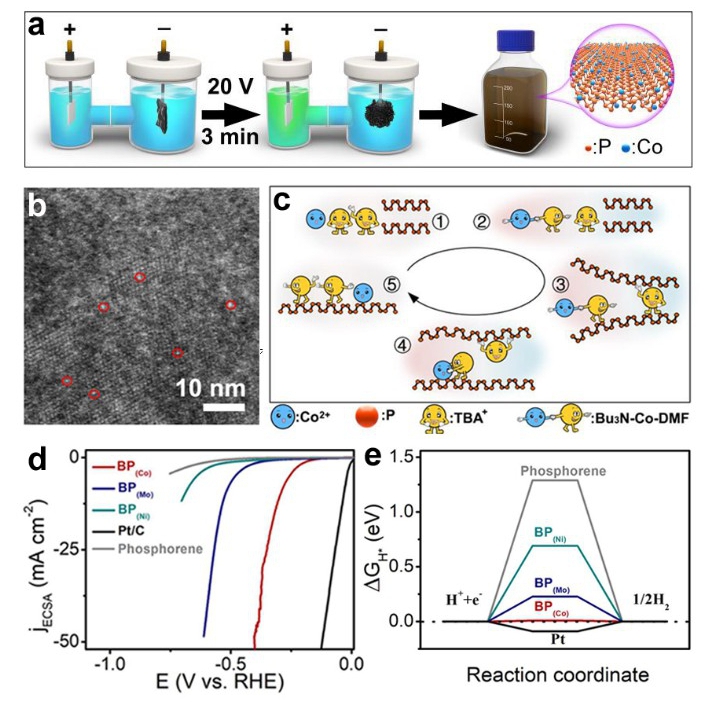
Fig. (a) Schematic diagram of the electrochemical system and synthetic procedure. (b) HAADF-STEM image and corresponding EDS elemental maps of BP (Co). (c) Schematic diagram showing the BP (Co) synthesis mechanism. (d) HER polarization curves of BP(Co), BP(Mo), BP(Ni), bare phosphorene, and Pt/C. (e) HER free energy ΔGH* for the hydrogen atom at the most stable site on the surface of bare phosphorene and BP(metal) (Image by Prof. YU Xuefeng)
CONTACT:
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn
Tel: 86-755-86585299